Carbon aerogel
Carbon aerogel is a new light and porous amorphous carbon material with a continuous three-dimensional network structure and adjustable density. The diameter of the colloidal network particles is 3-20nm, the pore size is less than 50nm, and the porosity is as high as 80%. ~98%, the specific surface area can reach 600~1100m2·g-1. In addition, carbon aerogel also has good electrical conductivity, with a conductivity of 10-25 S·cm-1. It is another ideal capacitor electrode material after activated carbon and activated carbon fiber.
1 Structure of carbon aerogel
In 1989, Pekala et al. of Lawrence Livemore National Laboratory in the United States used resorcinol (R) and formaldehyde (F) as raw materials to prepare organic aerogel (RF aerogel) by sol-gel method under the catalysis of sodium carbonate. ) and then carbonized in an inert atmosphere to obtain the first carbon aerogel (CRF). Both RF and CRF are composed of a large number of nano-scale three-dimensional network structures. Before carbonization, the aerogel clusters are of different sizes and large pores, which is a typical disordered porous structure; after carbonization, the colloidal particles become uniform and arranged. It is more compact and the pores become smaller, but the network structure and pore connectivity of the original aerogel are completely retained, as shown in Figure 2-5 by transmission electron microscopy.
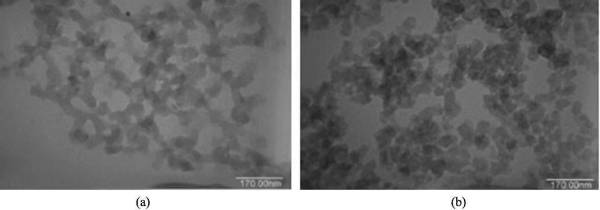
Figure 2-5 TEM images of aerogel (a) and carbon aerogel (b)
In the three-dimensional porous structure of CRF, there are three size characteristics: ①Single gel particle, with a size of 3-10nm; ②The mesopores formed between particles, with a size of 100-500Å (1Å=0.1nm); Pores, less than 10Å in diameter. During the preparation process, the catalyst content controls the particle diameter of CRF; the ratio concentration of the solution determines the density of CRF; the pyrolysis temperature determines the final microstructure of CRF, especially the particle size and pore size distribution.
2 Properties of carbon aerogel
2.1 Electrical properties
CRF is the only aerogel with conductivity, and its conductivity is 10-25S·cm-1. Its electrochemical performance is mainly related to density, temperature and particle size of carbon particles. Due to the large specific surface area, controllable pore size distribution and low resistivity of CRF, the use of carbon aerogels as binder-free electrode materials has a broad research space.
2.2 Thermal properties
CRF has excellent heat insulation performance, and its heat conduction is composed of three ways: gaseous heat conduction, solid heat conduction and radiation heat conduction. The thermal conductivity of the gaseous state decreases with the increase of the density and increases with the increase of the average pore size. However, due to the nanoporous structure of CRF, the thermal conductivity of the gaseous state is very small under normal pressure. For the evacuated CRF, the heat conduction mainly consists of Determined by solid-state heat conduction and radiative heat conduction. The solid-state thermal conductivity of CRF is 2 to 3 orders of magnitude lower than that of the corresponding glassy material, only about 1/500 of the thermal conductivity of the non-porous glassy material, and it increases with the increase of density, and it is connected with the particles related to closeness. The radiation heat conduction of CRF is mainly determined by infrared absorption, adding an appropriate amount of infrared absorber can effectively reduce the radiation heat conductivity. Under a certain density and microstructure, the thermal conductivity of CRF can be as low as 0.012W·m-1·K-1, which can be used as a high-temperature heat insulation material.
2.3 Optical properties
The CRF synthesized in the range of pH=2.0~3.0 has good light transmittance, and the light transmittance is significantly affected by the content of precursor and gelation time. The study found that the CRF with a density of 0.07g·cm-3 has a directional hemispherical emissivity of only 0.3% in the region of 40000-700cm-1, indicating that the carbon particles on the rough inner and outer surfaces of the CRF have strong light absorption. Zhang et al. found that the microstructural characteristics and density of CRF have an important influence on the microwave absorption characteristics. The reflection coefficient of CRF prepared with different catalyst concentrations has certain differences. When the gel becomes thicker, the maximum reflection value moves to low frequency.
2.4 Adsorption performance
CRF has a large specific surface area, abundant pores, and low density, which make it have a strong adsorption capacity for adsorbates. At the same time, CRF has stable chemical properties and generally does not react chemically with adsorbate, so it is the most promising adsorption material. In addition to strong adsorption properties for heavy metal ions such as Cd2+, Cu2+, Mn2+, Pb2+, etc., the adsorption capacity for organic vapors such as tetrahydrofuran, acetone, benzene, and carbon tetrachloride is also significantly higher than that of activated carbon.
3 Preparation of carbon aerogel
The research on CRF has a history of nearly 30 years. During this period, carbon aerogels have been made into different forms such as block, powder, microsphere, thin layer, and macroporous. But no matter how the shape is changed, its preparation process generally includes sol-gel process, drying process and carbonization process.
3.1 Sol-gel process
The sol-gel method is to use compounds containing highly chemically active components as precursors, uniformly mix the raw materials under liquid phase conditions, and perform hydrolysis and condensation reactions to form a stable transparent sol system. Slowly aggregated to form a gel with a three-dimensional space network structure. Taking the typical resorcinol-formaldehyde carbon aerogel as an example, resorcinol (R) and formaldehyde (F) are mixed at a molar ratio of 1:2, and under the action of catalyst Na2CO3, electrophilic substitution reaction occurs rapidly, Form single/multiple hydroxymethyl resorcinol, and then polycondensation reactions occur between the hydroxymethyl group and the unsubstituted position on the benzene ring, and between two hydroxymethyl groups, respectively forming methylene bonds (— The basic colloidal particles connected by CH2—) and methylene ether bonds (—CH2OCH2—), the basic colloidal particles continue to grow into clusters, and the clusters are further condensed to form a network polymer, that is, organic hydrogel. The reaction process is shown in Figure 2-6.
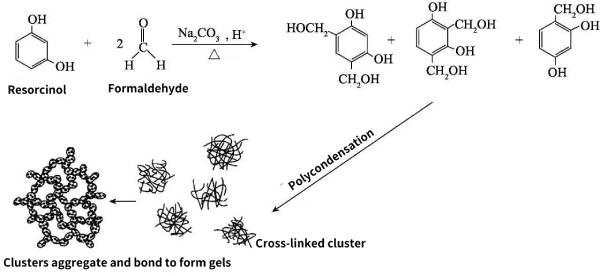
Figure 2-6 Formation of resorcinol-formaldehyde carbon aerogel
During the gelation process, the molar ratio of resorcinol to catalyst is an important parameter, which controls the density, porosity, specific surface area and other characteristics of organic aerogels. In addition, this ratio and the concentration of reactants also determine the time for the sol to form a gel, that is, the gelation time of the system.
3.2 The aging process of the gel
After the gel is formed, the structure and properties of the gel continue to change, such as the increase in pore size, the thickening of the network, and the decrease in specific surface area, while maintaining the liquid environment in the pores. This process is called the aging of the gel. During the aging process, the gel will undergo polycondensation, synergy, shrinkage and coarsening. The more thorough the aging process, the less shrinkage the gel dries.
The three main parameters affecting the gel aging process are aging time, temperature, and pH value. The longer the aging time, the larger the pores, but if the aging time is too long, the specific surface area of the aerogel will decrease. An increase in the aging temperature leads to an increase in the density of the resulting aerogel and a decrease in the specific surface area. The addition of acid can neutralize the excess alkali catalyst in the gel formation process, and at the same time promote the further cross-linking, aging, and increase the strength of the gel, and prevent the aerogel from cracking during the drying process.
3.3 Gel drying process
The drying of the gel is a crucial and difficult step in the sol-gel process, because the gel is prone to bending, deformation and cracking due to capillary tension, osmotic tension, separation tension and humidity tension during the drying process. Improving the network strength, improving the pore structure, and reducing the drying stress can prevent the gel from cracking during the drying process. Generally, measures can be taken: ① Change the synthesis conditions or add modifiers to increase the pore size of the gel, which can effectively reduce the capillary tension; ②Add surfactant to make the surface of the gel hydrophobic; ③Use solvent replacement to make the liquid in the gel pores into a liquid with low surface tension, reducing the stress generated during drying; ④Adopt a drying method with a short drying time to reduce the effect of drying stress time.
(1) Supercritical drying Supercritical drying is the earliest and most effective method of research. It makes the temperature and pressure of the gel system above the critical point of the solvent to form a supercritical fluid. This fluid has a strong ability to dissolve low-volatility substances, and its density and solvation power are close to liquids, but its diffusion properties and viscosity are close to those of gases. Due to the disappearance of the gas-liquid interface, the damage of the surface tension of the liquid to the gel nano-network structure during the gel drying process is avoided, and an aerogel that maintains the basic network structure of the gel is obtained, but the operation of supercritical drying is complicated and expensive. .
(2) Drying at normal pressure Compared with the supercritical drying process, due to the existence of surface tension, the dried gel often has a small porosity and a high density. If the pore size of the gel is increased by changing the synthesis conditions or adding an organic modifier, the capillary tension in the drying process can be effectively reduced, the drying shrinkage rate can be reduced, and an aerogel with high porosity and large specific surface area can be obtained, thereby greatly simplifying the process. The aerogel preparation process reduces its preparation cost. Although drying at normal pressure will cause shrinkage of pores, it is the simplest and most economical method.
(3) Freeze-drying In the late 1980s, Klvana et al. proposed to dry aerogel materials by freeze-drying, which is a method that can avoid obvious surface tension changes at the gas-liquid interface. The operation process is simple and the cost is low. This method is suitable for the drying of powdered aerogel. When the solvent content of the gel is high, the growth of solvent crystals during the freezing process will destroy the original network structure of the gel. In addition, the solvent crystals in the small pores melt and evaporate during the drying process, which will also cause the corresponding network to shrink and collapse. For bulk aerogels, they are easily fragmented after drying. Therefore, only when the gel pore size is large, the solid content is high and the original shape is not required to be maintained, the freeze-drying method is suitable.
(4) High-temperature carbonization process Organic aerogel becomes CRF with low resistivity after high-temperature carbonization in an inert atmosphere, and the stable network structure of the aerogel can be maintained after carbonization. The carbonization process is a high-temperature pyrolysis process. The functional groups inside are broken and released in the form of gas, forming a large number of new pores and increasing the specific surface area. During the carbonization process, the carbonization temperature, heating rate, time, and carrier gas flow will all affect the carbonization effect, affect the specific surface area and structure of CRF, and even affect its performance.




