1 Physical properties of aluminum metal and capacitor manufacturing
Before we explore the microscopic crystal structure of aluminum metal, we first introduce its macroscopic physical properties. From the theory of material structure, we know that all metals appear as crystals in the solid state. The basic particles that constitute the crystal lattice are atoms, and the bonds that connect the particles are metal bonds. The so-called metal bond refers to a type of chemical bond in which electrons in solids must be able to convert freely. Therefore, the atoms in the metal crystal lattice composed of metal bonds are actually composed of positively charged ions and negatively charged electrons. The ions are at the nodes of the lattice, and the valence electrons in the metal are not unique to any atom, but belong to the entire crystal and can move freely in the lattice, like a kind of “electron gas”, which makes aluminum metal have excellent conductivity and processing characteristics in capacitor manufacturing, as shown in Figure 1.
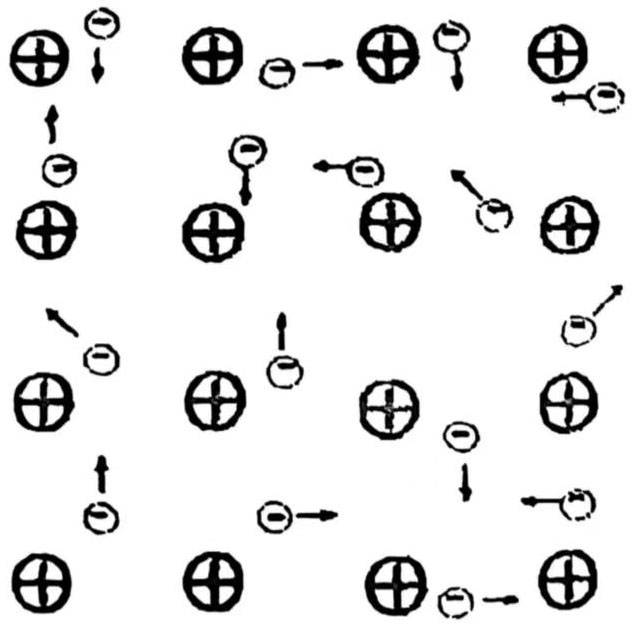
Figure 1 Metal bond diagram
Because there are electrons that can move freely in metals, this determines the inherent properties of metals, such as high electrical conductivity, thermal conductivity, brightness and good ductility (elasticity). These properties make metals, especially aluminum metal, have important applications in capacitor manufacturing. It should be pointed out that for metal crystals, pure metal bonds (completely free electrons) are ideal conditions, which are only possessed by certain metal single crystals. It can be imagined that the improvement of metal solidity may lead to the emergence of covalent bonds in addition to metal bonds. When the metal is deformed, the metal bonds can absorb the energy of deformation and partially become covalent bonds, causing the elasticity and ductility of the metal to decrease, and the hardness of the metal to increase.
The chemical symbol of aluminum is Al, the atomic weight is 27.0, and the atomic number is 13, indicating that it has 13 electrons. The number of its outermost electrons is 3, so when ionized, it can lose 3 electrons to become a trivalent cation.
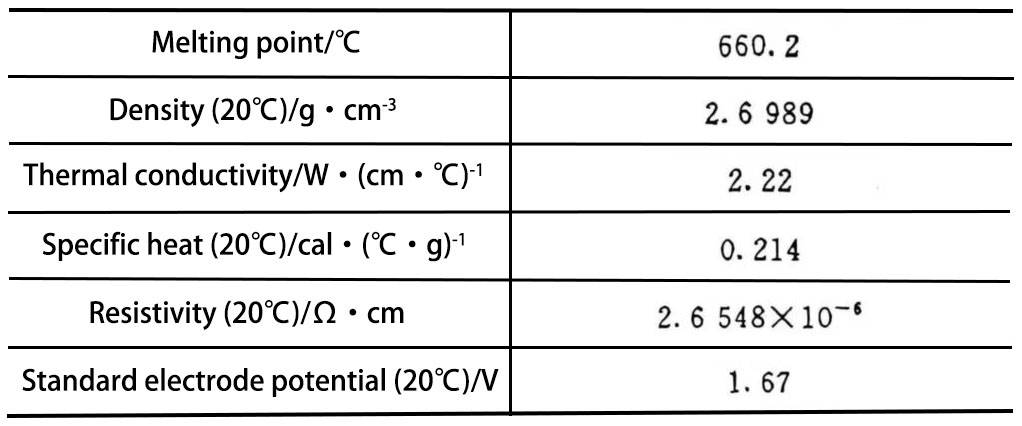
Metal aluminum is a white light metal with the characteristics of light weight, easy processing, good electrical and thermal conductivity, and strong corrosion resistance. Table 1 lists the physical and chemical properties of aluminum metal with a purity of 99.99%.
Table 1 Physical and chemical properties of aluminum
2 Crystal structure of aluminum metal
(1) Introduction to the manufacture of aluminum ingots
Before introducing the crystal structure of aluminum metal, let’s briefly describe the manufacture of aluminum ingots and aluminum foil processing. As we all know, my country has a large amount of aluminum vanadium containing 50%~70% alumina hydrate (AI2O3·nH2O), which is the raw material for smelting aluminum ingots.
The refining of aluminum metal is divided into two stages:
The first stage is to produce high-purity aluminum oxide, also known as bauxite or aluminum oxide (AI2O3). It first needs to treat bauxite (AI2O3·nH2O) with sodium oxide (NaOH) at high temperature → add water (H2O) to dilute → filter to separate the insoluble red mud and dissolved components, the latter is sodium aluminate (NaAIO2) solution → then add a small amount of sodium hydroxide as a crystallization nucleus to make the dissolved aluminum turn into aluminum hydroxide white mud precipitate → after roasting, the water evaporates to obtain white powder aluminum oxide.
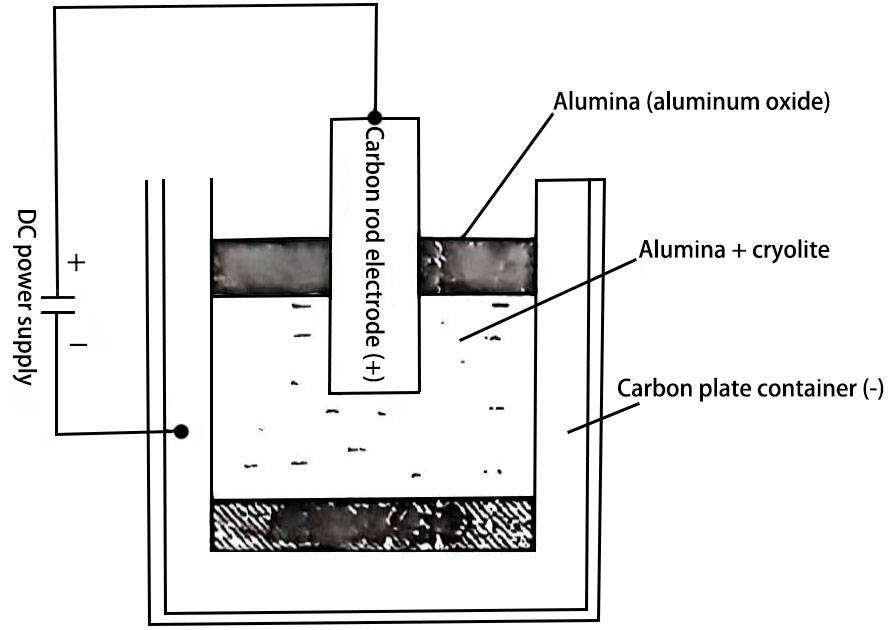
Figure 2 Schematic diagram of alumina electrolysis principle
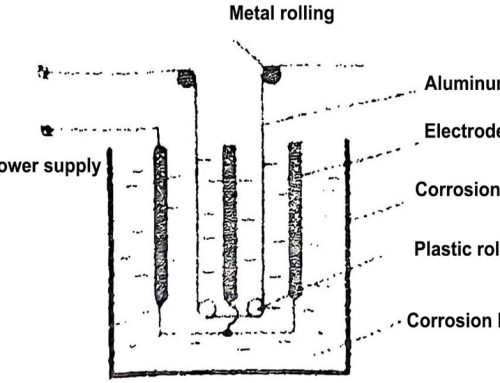
In the second stage, the aluminum and oxygen in aluminum oxide (AI2O3) are separated by electrolysis. In order to save energy and make the electrolysis process easy to implement, non-aqueous solvent cryolite (AIF3·3NaF or Na3AIF6) is used to dissolve alumina at 900~1 000 ℃. The electrolysis principle diagram is shown in Figure 2. The metallic aluminum liquid obtained by this method becomes a primary aluminum ingot after casting, and its pure square lattice structure is about 99%. This method is widely used in the field of capacitor manufacturing, especially in the production of aluminum electrolytic capacitors. In order to obtain high-purity aluminum (purity above 99.9%), the primary aluminum ingot is further purified and refined. To produce 1 ton of primary aluminum ingot, about 2 tons of bauxite are needed, that is, 4 tons of bauxite are needed, and the power consumption is about 20,000 kilowatt-hours.
(2) Crystal structure of aluminum metal
From metal physics, it is known that the crystal structure of aluminum metal is a face-centered cubic lattice, as shown in Figure 3. Its unit lattice can be regarded as a regular hexahedron, which has 8 corners and 6 faces with 6 center points, so there are 14 places with an aluminum atom each. If a large number of face-centered cubic crystals are continuously gathered together (that is, one face of a lattice also serves as a face of another lattice), an aluminum metal crystal is formed. The crystal structure constants of gold aluminum are shown in Table 2.
Table 2 Constants related to the crystal structure of metallic aluminum
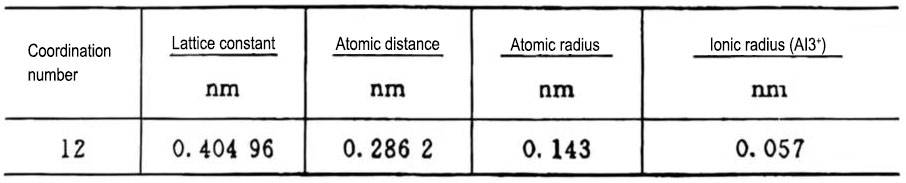
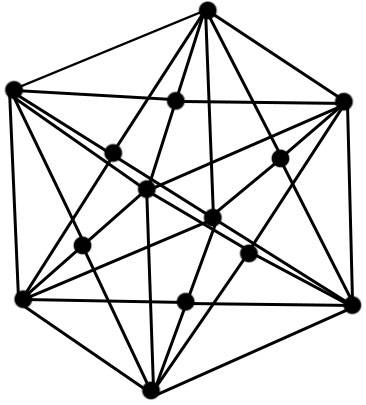
Figure 3 Lu’s face-centered cubic crystal structure
(3) Mechanical processing and changes in aluminum crystal structure
Aluminum has good metallic ductility, so it can be processed by mechanical rolling and several roller rolling processes to form raw foils of various specifications for aluminum electrolytic capacitors.
The face-centered cubic aluminum crystal structure shown in Figure 3 is an ideal lattice state. In fact, it is difficult to achieve the absolute ideal purity of aluminum ingots during refining and purification. Metal impurities such as Fe, Cu, Mn and non-metallic impurities such as Si are always inevitable. In addition, the cooling method of aluminum ingots after casting not only affects the coarseness of aluminum crystal grains and the arrangement of lattice points, but also causes lattice point defects. Lattice defects refer to the area where the structure of the actual metal crystal deviates from the ideal point structure.
It should be pointed out here that during the rolling process of aluminum metal at room temperature, the aluminum crystal will undergo irreversible plastic deformation, that is, the metal atoms at the lattice nodes will produce relative slip under the action of mechanical external force, causing the distance between the metal atoms to change, resulting in the distortion of the lattice, and may cause the original grains to break into many small grains. This will lead to changes in the shape and size of the aluminum foil, and the foil will become harder (caused by internal stress), so annealing treatment is usually required to eliminate internal stress and improve lattice defects, and make the small grains grow. This process is crucial for the quality control of aluminum electrolytic foil in capacitor manufacturing.