1 Effect of Aluminum’s Corrosion Resistance on Capacitor Performance
1.1 Relationship between aluminum corrosion resistance in the atmosphere and capacitor performance
Aluminum is usually very corrosion-resistant under atmospheric conditions. Through experiments, it was found that aluminum quickly formed an oxide film with a thickness of about 3nm in dry oxygen, after which oxidation was almost stopped. Even if aluminum was placed in a humid environment, its surface would initially undergo relatively obvious oxidation, but the oxidation rate would soon slow down and stabilize. The corrosion resistance of aluminum is closely related to the oxide film formed on its surface, which is particularly important for capacitor performance, because the stability of the oxide film directly affects the insulation and service life of the capacitor. According to data reports, when the aluminum metal surface is heated in air, an amorphous aluminum oxide film is formed below 450℃, and crystalline γ-Al2O3 is formed above 450℃.
In pure water, aluminum is actually not corroded, but if the water contains alkaline impurities, the corrosion rate of aluminum increases significantly. In aqueous solutions containing chloride ions that can destroy the oxide protective layer of aluminum and salts such as mercury and copper, the corrosion rate of aluminum will also accelerate.
1.2 Behavior of Aluminum on Inorganic Acids
The effects of inorganic acids on aluminum are extremely complex. Aluminum is extremely stable in concentrated nitric acid at room temperature, but it will be destroyed very quickly in dilute nitric acid; dilute sulfuric acid (concentration less than 10%) has very weak corrosion on industrial aluminum at room temperature, but with the increase of concentration and temperature, the corrosion rate increases significantly. Aluminum is actually stable in 100% sulfuric acid; hydrochloric acid causes aluminum to be destroyed quickly, and it is more serious at high temperatures. These corrosion characteristics of aluminum may affect the capacitor performance, especially in harsh environments, the stability of capacitors may be affected. The reaction is as follows:
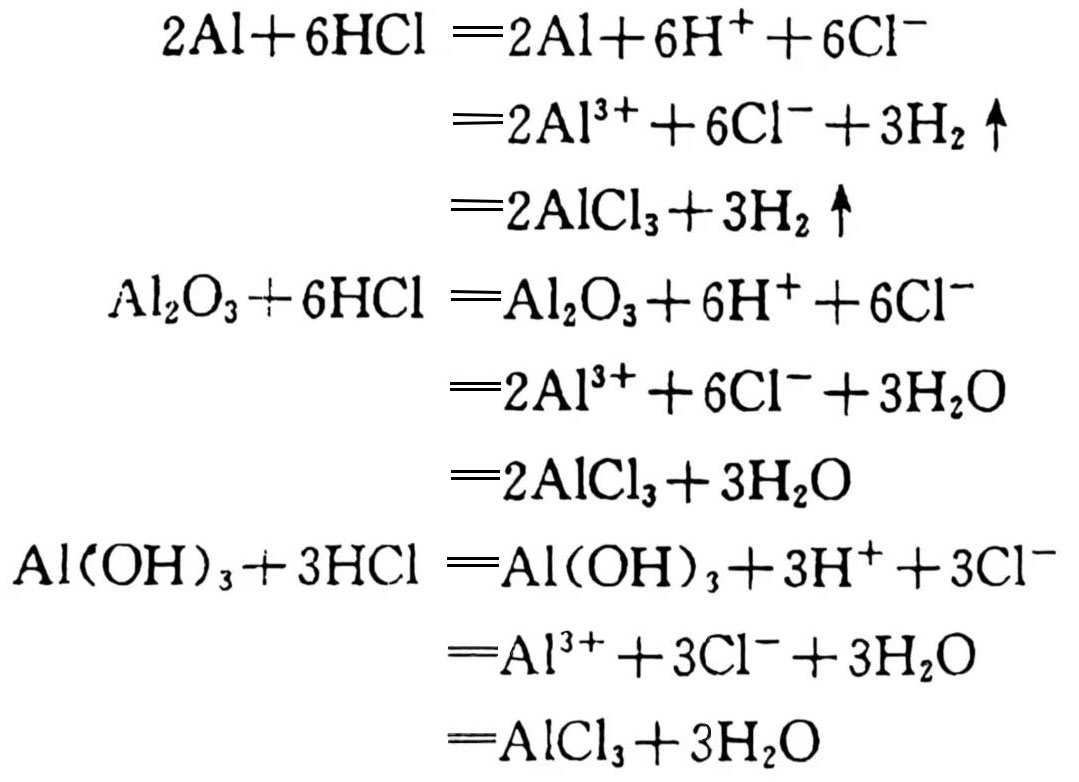
Hydrofluoric acid has the same effect on aluminum as hydrochloric acid. However, dilute phosphoric acid (concentration less than 1%), chromic acid (concentration 10%) and various concentrations of boric acid solutions have little effect on aluminum. The capacitor performance is affected by external chemicals during use, especially when aluminum is one of the capacitor components, the performance may be degraded due to the corrosion of these acidic substances.
1.3 Behavior of aluminum towards mixed inorganic acids
Adding glycerol, ethylene glycol and the like to the acid can inhibit the reaction, but the reaction of aluminum in some inorganic acid mixtures is more complicated. D. Altpenpohl found in his study on the properties of aluminum surface oxides:
① Phosphate monochromic acid solution
20 g CrO3+35 ml 85% H3PO4+1 000 ml H2O, it can quickly dissolve AI(OH)3 and γ1-Al2O3 at 85℃, but almost does not dissolve γ-Al2O3 and aluminum.
② Bromine methanol solution
The bromine methanol solution is prepared by dissolving a 10% bromine solution in methanol. It almost does not dissolve γ-Al2O3 at high temperatures, but only dissolves aluminum.
1.4 Behavior of aluminum towards inorganic alkali
Aluminum is destroyed in caustic alkali (such as NaOH, KOH solution). The reaction generates hydrogen and aluminate:
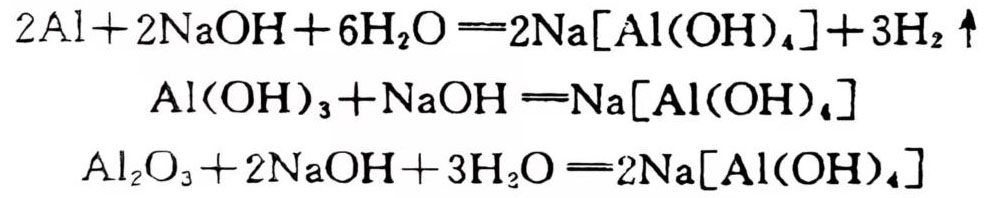
Comparing the behavior of aluminum in alkaline solution and acid solution, alkaline solution dissolves aluminum in a plane form, so if you want to get deep corrosion pits, you can’t use alkali. Generally, alkali is used to clean the aluminum surface to remove residual oil. If alkali is used as a detergent but you don’t want to corrode aluminum, you can add sodium silicate and sodium chromate as inhibitors, which are very effective.
1.5 Reaction of aluminum with salt
Chloride (salt) is most likely to corrode aluminum, because the chloride ion (CI–) has a small ion radius, strong migration ability, and strong affinity, so it is easy to invade the interior through the structural defects of the oxide film and the hydroxide film. However, there are also some salts that can protect the oxide film and hydroxide film and inhibit dissolution, such as adipates, borates, phosphates and silicates. For this reason, these salts can be used as solutes in the working electrolyte to improve the stability of the capacitor performance.
1.6 Reaction of aluminum and organic acids
The organic acids mentioned here mainly include acetic acid, butyric acid, citric acid, propionic acid, etc., which have little effect on aluminum. However, formic acid and other organic acids containing chlorine are exceptions.
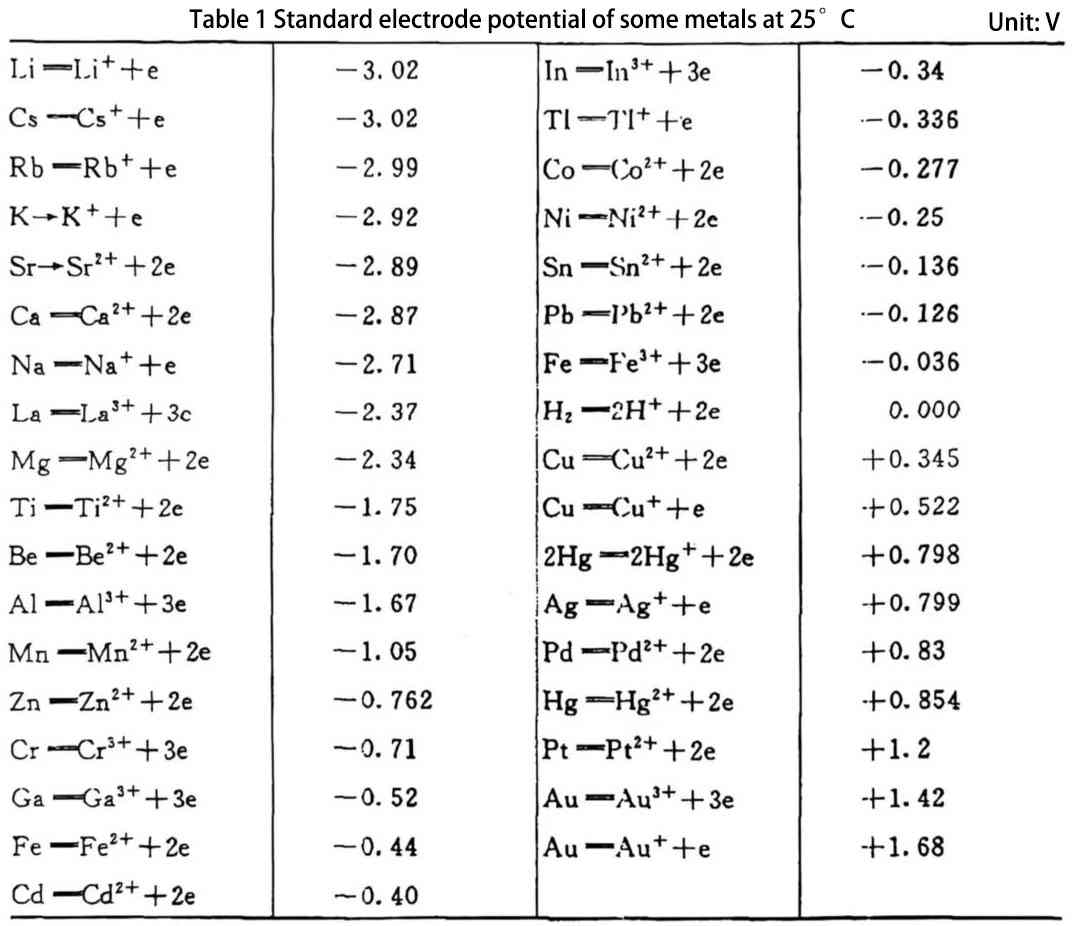
2 The effect of impurities on the physical and chemical properties of aluminum
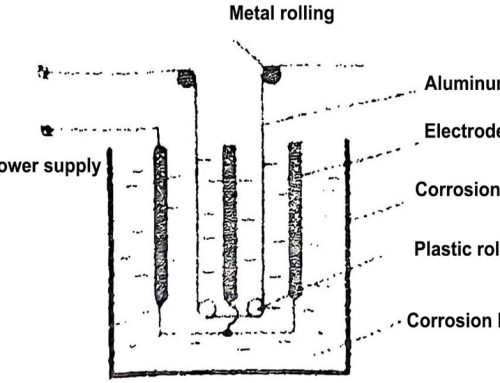
Impurities have a significant effect on the electrical conductivity, thermal conductivity, mechanical properties and corrosion resistance of metallic aluminum. For the anode aluminum foil used as an aluminum electrolytic capacitor, it will also affect the corrosion coefficient, formation characteristics, and even the electrical properties and life characteristics of the product. The main impurities in aluminum are Fe, Cu, Si, etc. If the electrokinetic series electrode potential of some of its impurities is positive than that of aluminum metal, then each impurity becomes the cathode of the corrosion microbattery for Al metal, and the aluminum metal adjacent to this impurity becomes the anode of the corrosion microbattery. Therefore, there must be many tiny cathode areas and anode areas coexisting on the entire surface, thus forming many tiny corrosion micro-batteries. Figure 1 shows a schematic diagram of aluminum foil containing impurities being corroded in an electrolyte solution.
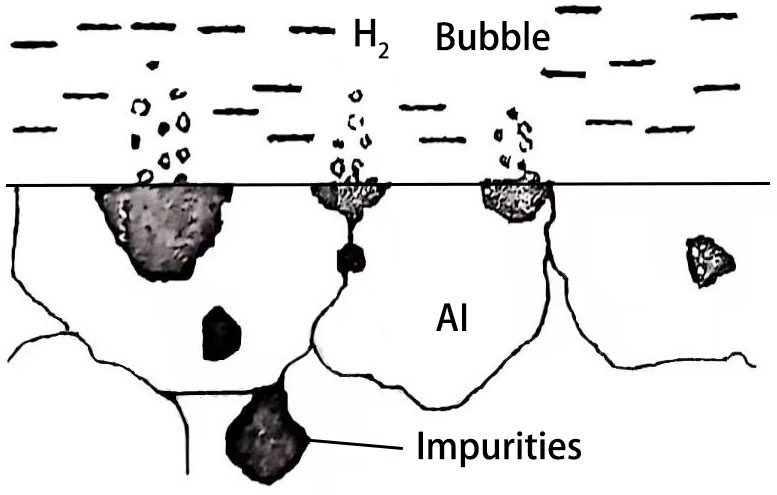
Figure 1 Schematic diagram of aluminum foil containing impurities being corroded in an electrolyte solution
This micro-battery corrosion undergoes an oxidation reaction at the anode (anodic process) and a reduction reaction at the cathode (cathode process) just like electrochemical corrosion, namely;
① At the anode, aluminum metal loses electrons and becomes aluminum ions that enter the solution at the interface:
Al→Al3++3e (anodic process)
② Electrons flow from the anode to the cathode through the junction;
③ At the cathode, the incoming electrons are gathered and adsorbed at the cathode/solution interface and captured by the substance (D) that can absorb electrons, namely
e+D→[D·e] (cathode process)
There are many substances that can combine with electrons at the interface between the cathode and the solution, but in most cases, they are H+ and O2 in the solution. H+ combines with electrons to form hydrogen H2, which escapes at the interface between the cathode and the solution.
2H++2e→H2↑
When oxygen is present in the solution, the so-called oxygen ionization process is carried out at the cathode, and oxygen is reduced to hydroxide (OH–) ions:
O2+2H2O+4e-4OH–
The former is usually also called hydrogen depolarization corrosion, and the latter is called oxygen depolarization corrosion. Some data point out that in the case of dissolution in the solution, if the potential of the anode metal is less than 0.805 volts (the equilibrium potential of the oxygen electrode), oxygen depolarization corrosion may occur.
From this, it can be seen that in the microbattery, the anode process is the corrosion process of metal aluminum. The metal electrode with high potential is the cathode of the corrosion battery, which only plays the role of transferring electrons and is not corroded; while the metal with low electrode potential becomes the anode of the corrosion battery. Moreover, the greater the absolute value of the difference between the anode electrode potential and the cathode electrode potential, the greater the tendency of impurities to affect corrosion. The poorer the purity of aluminum, the worse the corrosion resistance. From the standard electrode potential of some metals in Table 1, it can be seen that the most serious impact is copper impurities, followed by iron impurities. Therefore, for long-life and high-quality aluminum electrolytic capacitors, high-purity aluminum must be used, especially anode foil.
In addition to affecting the corrosion of aluminum foil, impurities in aluminum foil also seriously reduce the formation speed of aluminum foil. For example, when the content of iron impurities in the anode foil increases, the formation speed decreases sharply, and when the content reaches 1%, it is actually impossible to form. Figure 2 shows the effect of the amount of iron impurities in the anode aluminum foil on the formation speed. In addition, Si in the impurities can prevent the formation of γ-Al2O3, while Mg promotes its formation and reduces the formation temperature. These changes will affect the capacitor performance, especially their stability and life during operation.
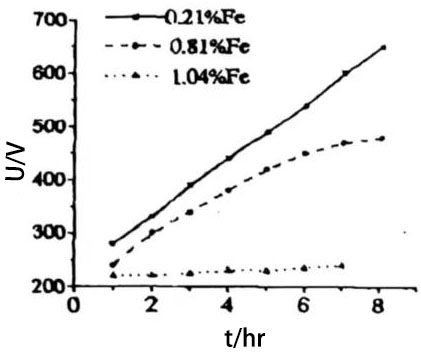
Figure 2 Effect of the amount of iron impurities in anode aluminum foil on the formation rate
3 Reaction of aluminum and water
When high-purity aluminum is immersed in pure water, no reaction will occur when the water temperature is below 30℃, but when the water temperature rises, water and aluminum will react to produce aluminum hydroxide and hydrogen. The generation of hydrogen indicates that when the water temperature is high, water molecules are ionized into H+ and OH–:
H2O →H++ OH–
Hydrogen ions absorb electrons from the aluminum surface and become hydrogen molecules to escape, while positively charged aluminum and negatively charged OH– combine to form aluminum hydroxide. Their reactions are as follows:
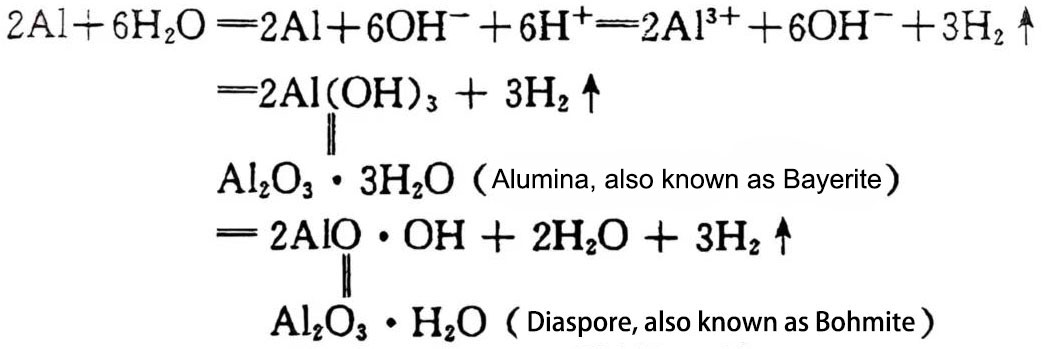
Aluminum hydroxide precipitates in water in the form of white colloid. Most of it forms a film on the aluminum surface and remains. When the film reaches a certain thickness, the reaction between aluminum and water weakens and almost stops. This reaction phenomenon has an important influence on the capacitor performance, especially in the production process of electrolytic aluminum foil. The oxide film formed on the aluminum surface will directly affect the working characteristics and life of the capacitor.
The reaction of aluminum and water to form aluminum oxide is different at different temperatures. The general situation is as follows:
① Below 20~30 ℃, the reaction rate is extremely slow;
② At 40~60 ℃, a relatively thick film is generated, the thickest is about 5μm, and the water content is about 35%~70%. It is composed of amorphous aluminum oxide (Al2O3·nH2O) and alumina (Al2O3·3H2O), and its specific gravity is 3.014 g/cm3;
③ At 70~80 ℃, the alumina content increases.
④ To the boiling point of about At about 98℃, the reaction mainly produces diaspore (AI2O3·H2O) and alumina (Al2O:·3H2O), each accounting for about half. The overall film water content is about 32%, and the specific gravity is 2.41g/cm3
⑤ The oxide film obtained by the reaction at 155~200℃ has the largest content of diaspore, and the water content accounts for about 15%.