It is well known that metal crystals are composed of neatly arranged metal ions (also called ions) and electrons (called electron gas) flowing between them. When metal aluminum is immersed in hydrochloric acid electrolyte, bubbles are observed to emerge from the liquid, the surface of the aluminum foil begins to be corroded, and aluminum particles are dissolved. In fact, there are always some structural defects, impurities, stress concentration, and areas such as different structural states of aluminum grains and grain boundaries on the surface of aluminum foil. These imperfect areas have different chemical potentials from other areas, which will cause the former to constitute the anode area of the corrosion cell, while the latter will become the cathode area of the corrosion cell. The affinity of aluminum cations in the anode area to electrons is weaker than that in the cathode area, so it is easy to lose electrons. In the presence of an electrolyte solution (such as a hydrochloric acid solution), the aluminum ions on the aluminum surface will be hydrated by the polar water molecules in the solution. Since the affinity between aluminum ions and electrons in the anode area is weak, the hydration energy generated during hydration is sufficient to overcome the attraction between aluminum ions and electrons in the aluminum lattice, so that the aluminum ions in the anode area on the aluminum foil are first separated and enter the wave phase in contact with the aluminum surface to form hydrated aluminum ions, which react with the chloride ions in the solution to form soluble aluminum trichloride AICL3. The electrons on the anode area lattice will pass through the cathode area in contact with it and be captured by the hydrogen ions (H+) at the interface between the cathode and the solution. The H+ will be reduced to hydrogen (H2) and escape. This process reflects the basic principle of the Capacitor aluminum foil corrosion mechanism.
The corrosion behavior of aluminum foil with metals such as Cu, Fe, Pd, etc., whose electrode potential is more positive than AI, in salt electrolyte solution is specifically reflected in the Capacitor aluminum foil corrosion mechanism:
Anode area:
![]()
In the solution at the metal/solution interface:
![]()
Cathode area:
![]()
From the above analysis, it can be seen that the starting points of corrosion are scattered disorderly on the aluminum surface. These starting points of corrosion mainly occur at defects, impurities, grain boundaries between grains, etc. on the aluminum surface. For cold-rolled aluminum foil, corrosion may also start from certain defects or locations where the plastic deformation lattice is distorted on the line parallel to the original rolling direction. In this way, corrosion starts from a local area on the surface of the metal foil, then spreads, and finally affects the entire surface.
It should be pointed out that in the corrosion process of aluminum foil, the aluminum in the anode area is continuously corroded during the corrosion process, while gas (H2) is continuously released in the cathode area. This metal corrosion in which hydrogen ions are reduced on the cathode and H2 is precipitated is also called hydrogen depolarization corrosion.
There are various methods to expand the surface area of aluminum foil, such as chemical corrosion, DC corrosion, AC/DC corrosion, AC corrosion, reaction exchange, etc. These methods all involve different aspects of the Capacitor aluminum foil corrosion mechanism.
1 Study on the chemical corrosion method of Capacitor aluminum foil corrosion mechanism
Because aluminum has amphoteric properties, it can be corroded in acid or alkali solution, but its corrosion form in alkali solution is mainly plane corrosion. Therefore, aluminum foil is usually not corroded.
Acid has a strong corrosive property, especially hydrochloric acid and its salts, which can corrode the oxide defect part on the aluminum surface and form the starting point of corrosion. In terms of its corrosion mechanism, it mainly includes solubility corrosion and local micro-battery corrosion. Therefore, in the 1950s and 1960s, most small-scale, hand-made electrolytic capacitor factories used this manual chemical etching method to corrode aluminum foil.
Since hydrochloric acid is a strong electrolyte, it is easy to ionize into hydrogen positive ions (H+) and chlorine negative ions (CI–) in the solution. When the aluminum foil is immersed in the solution, based on the above corrosion mechanism, many corrosion micro-batteries will first be formed at the sensitive points or areas where corrosion occurs, causing the aluminum metal in the anode area of the micro-battery to continuously dissolve and react with CI– in the solution to generate soluble aluminum trichloride (AICl3), while gas (H2) will escape in the cathode area, that is:
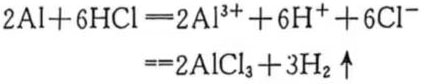
The above reaction will reduce the corrosion rate as the acid concentration decreases and the concentration of the product aluminum salt increases, and eventually it will be very slow. This method is not suitable for intensive large-scale production.
In fact, there are always some metal impurities in aluminum foil to a greater or lesser extent, and sometimes even some impurities are deliberately added to improve the corrosion morphology and performance of the corrosion foil. In the presence of electrolyte solution, these impurities and aluminum gold form a corrosion micro-battery. If the standard electrode potential of the impurity is more positive than that of aluminum, the aluminum in contact with the impurity becomes the anode of the corrosion micro-battery, the aluminum is corroded, and the impurity becomes the cathode, and H2 escapes at the interface. Therefore, in these local areas, the corrosion rate of aluminum is much faster than other uniform surfaces, resulting in different corrosion rates at different locations on the aluminum surface, which is conducive to expanding the specific surface area. This corrosion process and mechanism are directly related to the Capacitor aluminum foil corrosion mechanism.
Of course, chemical corrosion using hydrochloric acid solution will have the problem of difficult cleaning after corrosion, because CI– and other impurities in the micro-corrosion pits are difficult to completely clean, which will affect the subsequent process and product performance. By understanding the Capacitor aluminum foil corrosion mechanism, more effective cleaning methods can be adopted during the corrosion process to reduce the impact on subsequent production and product performance.
2 DC corrosion method
DC corrosion method, also known as electrochemical corrosion method, places aluminum foil in a hydrochloric acid solution with a carbon rod as the negative electrode, the aluminum foil is connected to the positive electrode of the power supply, and the carbon rod is connected to the negative electrode, as shown in Figure 1. The corrosion rate will be related to the current density flowing through. In this way, the corrosion parameters such as current density, solution concentration, solution type and liquid temperature can be appropriately selected, and these parameters can be determined according to the different requirements of the corrosion foil. Therefore, this corrosion method is still widely used to date. Most of the anode aluminum foils for high voltage and flash lamps use the DC corrosion method. The disadvantage of this method is that the corrosion uses low voltage and high current, the contact point is prone to flash, and there are many faults.
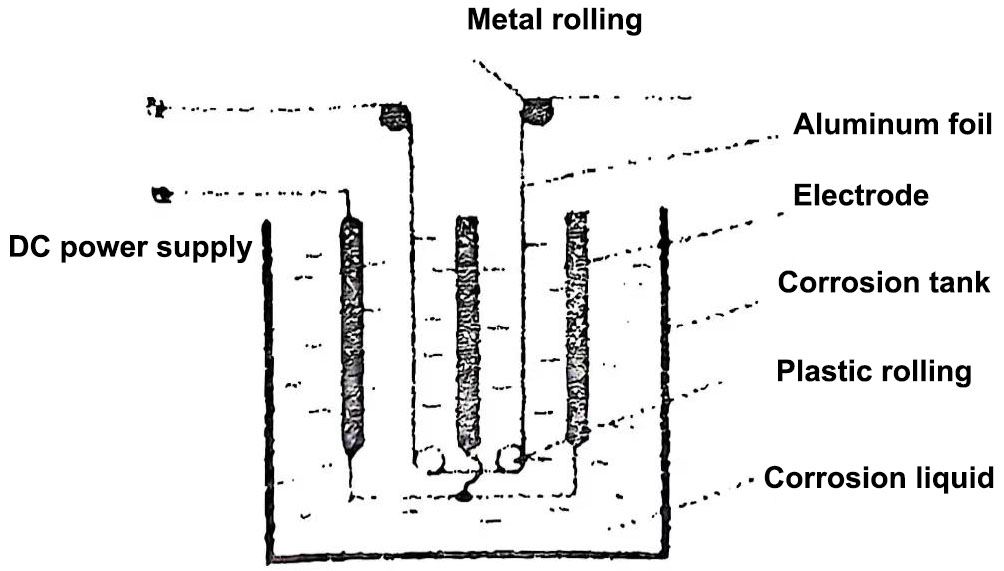
Figure 1 Schematic diagram of DC corrosion of aluminum foil
The DC corrosion mechanism is currently most commonly explained by the tunnel corrosion theory. In the early stage of pitting, a semi-cubic pit is formed. The cubic pit is generated perpendicular to the metal surface along the [100] crystal plane. The pit diameter is maintained at about 1μm, the surface density is about 105~106/cm2, and the pit depth is 50~100 μm. This pit is like a tunnel, and people call this corrosion tunnel corrosion (Tunnel etch). The dissolution of metal in tunnel corrosion starts from the front surface of the tunnel. When the cubic pit is formed to a certain critical size, the side wall of the pit no longer dissolves, while the front crystal surface continues to dissolve, thus forming a tunnel.
The cross-section of the anode aluminum foil with tunnel corrosion was photographed using a scanning electron microscope, and the results are shown in Figure 2. In the figure (a), the tunnel does not penetrate the anode foil. Since both the upper and lower surfaces are corroded, there is a residual part in the middle that has not been corroded, thereby enhancing the mechanical strength of the aluminum foil. Figure (b) is a tunnel-penetrating anode foil, with no residual part in the middle and poor mechanical strength.
The factors affecting tunnel corrosion include: the composition and temperature of the etching solution, the electrocorrosion time, the polarization potential or current, the surface state of the aluminum foil and the crystal structure. Among them, the polarization voltage or current, the CI– content in the etching solution and the corrosion temperature have the greatest impact. The level of polarization potential has a significant impact on the pit density, the pit growth direction and growth rate and the tunnel length. As the liquid temperature increases, the tunnel generation rate rises linearly, and the tunnel width becomes narrower. The study of the Capacitor aluminum foil corrosion mechanism reveals the important role of these factors in the corrosion process of aluminum foil.
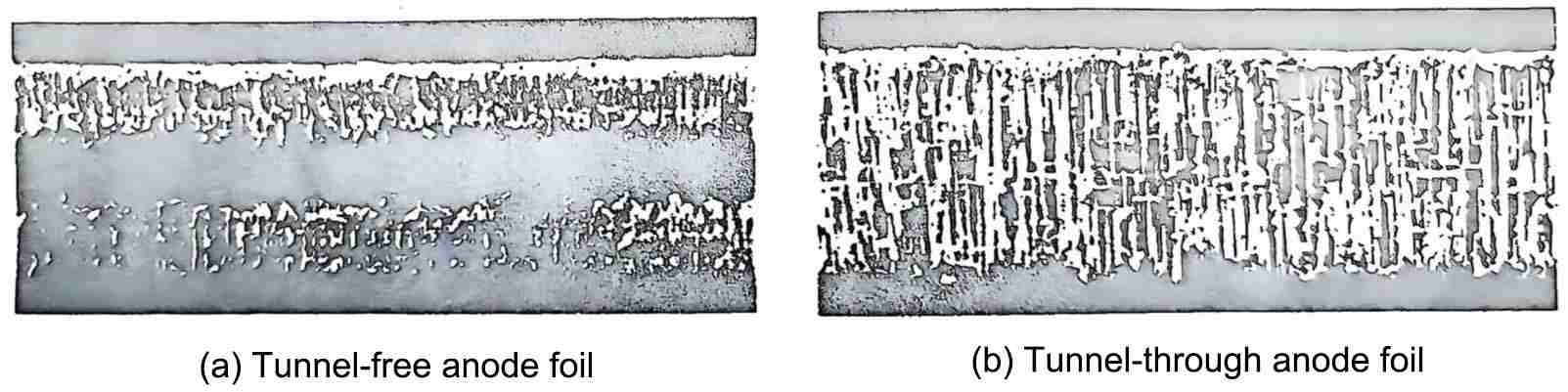
Figure 2 Scanning electron microscope image of the etched foil (300 times)
3 AC corrosion method
The principle diagram of the AC method is shown in Figure 3. The corrosion principle is basically the same as the DC corrosion method, except that the aluminum foil is corroded when it is in the positive half cycle of AC, and it does not corrode when it is in the negative half cycle.
AC corrosion can produce high-density uniform fine pits on the surface of the aluminum foil, and can obtain the largest specific surface area of the etched foil, so it is widely used in low-voltage etched foil.
In the Capacitor aluminum foil corrosion mechanism, when aluminum is AC-corroded in hydrochloric acid solution, the positive half-wave is the pitting initiation and generation period, the pitting nucleation is at the weak point of the structure, and the corrosion has a crystal orientation, and the small pits are square. As the frequency of AC increases, the surface pit density increases, the diameter decreases, and the distribution of the pits is more uniform. In the negative half cycle of current, aluminum is in a cathodic polarization state, and a protective film (AI(OH)3/AIOOH) different from the anode passivation film will be formed in the pit. The formation of the protective layer may be due to the increase in the pH value of the solution near the aluminum surface caused by H+ discharge, resulting in the precipitation of AI(OH)3, or it may be due to the hydration of the original oxide film on the aluminum surface.
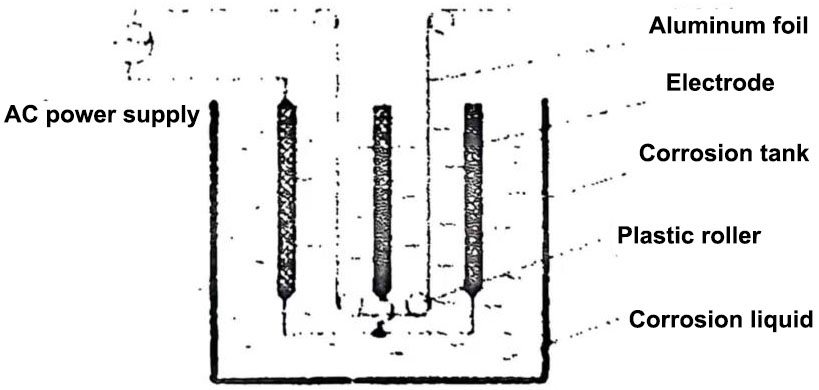
Figure 3 Schematic diagram of AC corrosion of aluminum foil
Nguyen and Foley believe that the pitting initiation process of aluminum foil in chloride solution is as follows:
① Adsorption of corrosive anions on the oxide film of aluminum foil.
②Chemical reaction between adsorbed anions and AI+ in oxide grains:
![]()
Or ![]()
③Dissolution and thinning of the oxide film on the surface of aluminum foil.
④Anions directly corrode the exposed metal aluminum, generate complexes and rapidly hydrolyze:
![]()
The factors affecting AC corrosion are the temperature of the corrosion solution, the corrosion time, the frequency of the corrosion current and the current density. When the temperature of the corrosion solution rises, the accumulation and chemical adsorption of CI– on the surface of the aluminum foil increase, which increases the number of points where the AI2O3 film is damaged, the number of pitting holes increases, the generation rate increases, and the pore size decreases. The effect of liquid temperature on the specific volume C0 of aluminum foil is shown in Figure 4.
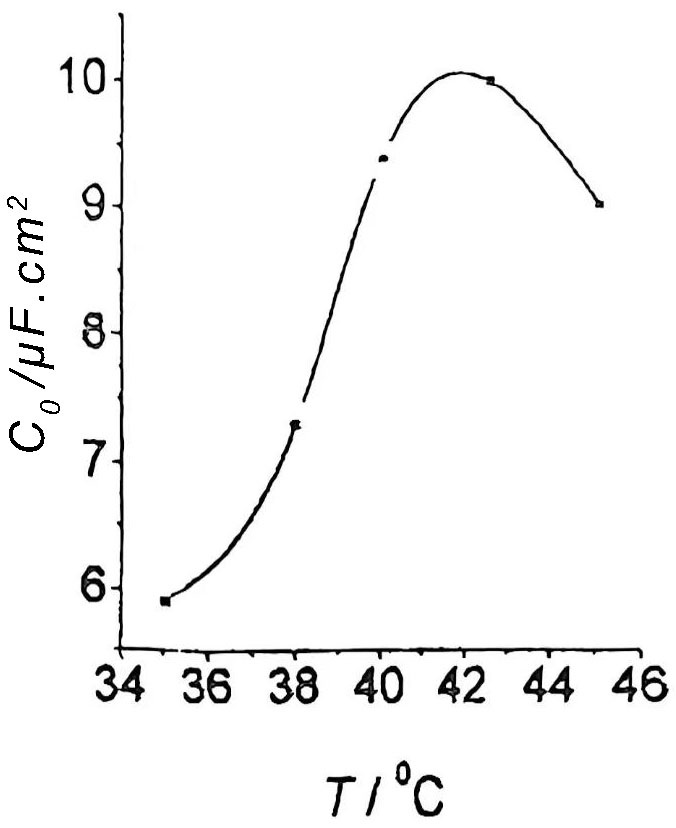
Figure 4 Effect of liquid temperature on the specific volume of aluminum foil
The frequency of the corrosion current also has a great influence. Experiments show that as the frequency increases, the pitting aperture decreases, the pit density increases, and the uniformity of the pit distribution improves, which is beneficial to the optimization of the Capacitor aluminum foil corrosion mechanism. When the frequency increases to a certain critical value, due to the small pitting aperture, the pits are easy to merge into large holes, resulting in a decrease in the specific surface area of the aluminum foil and a decrease in the specific volume C0.
As the current intensity increases, the specific volume increases, but when the current is too large, the pits are generated too quickly, which easily causes the corrosion film to fall off, resulting in a decrease in the specific volume C0.
As the corrosion time increases, the pits grow fully, but if the corrosion time is too long, the aluminum foil is thinned and the purpose of expanding the surface area cannot be achieved. This situation is as shown in Figure 5.
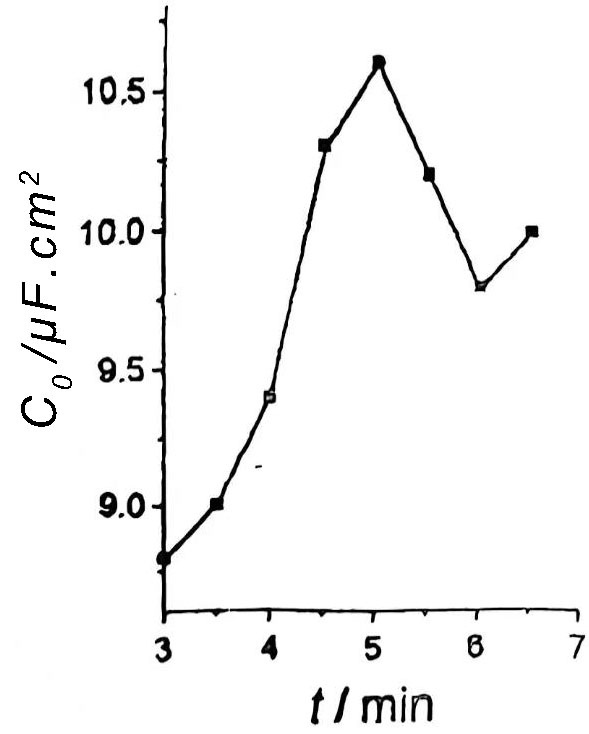
Figure 5 Relationship between corrosion time t and aluminum specific volume C0
Therefore, there are many factors that affect the corrosion quality of aluminum foil. It is necessary to comprehensively consider the influence of corrosion solution formula, corrosion solution temperature, corrosion time, power supply frequency and current density, and optimize the corrosion process parameters.
Advantages of AC corrosion method:
① Save huge high-current rectifier equipment.
② Eliminate various problems easily caused by poor contact and high current between the conductive roller of the corrosion linkage machine and the aluminum foil.
③ The corrosion holes are uniform and the corrosion effect is good.
④ The center of the foil cross section is not corroded through, and the core remains, so a corrosion foil with high mechanical strength can be obtained.
Disadvantages of this method:
① The holes are fine, and it is difficult to obtain high-voltage corrosion foil with a thicker oxide film.
② It is more troublesome to clean the roots after corrosion.
Comprehensive analysis of its advantages and disadvantages shows that it is more suitable for low-voltage aluminum foil to adopt the corrosion method based on AC corrosion.
4 AC/DC corrosion method for Capacitor aluminum foil corrosion mechanism
This method aims to overcome the shortcomings of DC corrosion method and AC corrosion method, and at the same time obtain corrosion foil with deep holes and large corrosion coefficient, which is particularly suitable for the production of high-voltage corrosion foil.
The AC/DC corrosion method can be a DC power supply with a certain amount of AC power superimposed on the DC, or it can be first etched with DC and then with AC.